2025 Volume No 50 – pages 20-32
Title: Nanocarrier-encapsulated SGLT-2 inhibitor (dapagliflozin) protects against diabetic cardiomyopathy by reducing myocardial fibrosis |
Authors: CY Li, F Xu, LR Zhu, C Cheng, JY Kuang, T Zhao |
Address: Department of Cardiology, The Second Hospital of Shandong University, 250033 Jinan, Shandong, China |
E-mail: zhaotongzbw at 163.com |
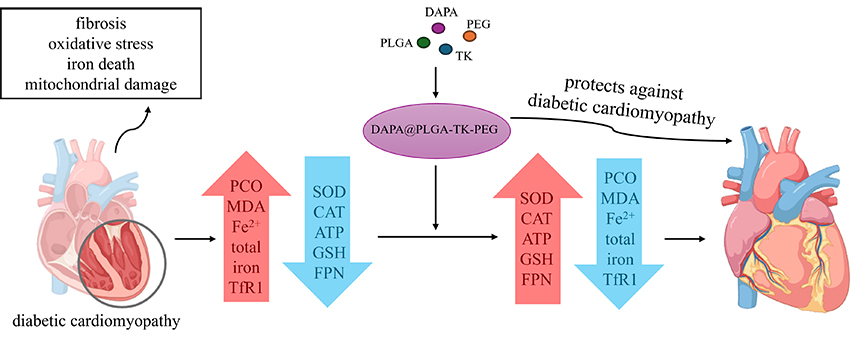
Abstract: Background: Diabetic cardiomyopathy (DCM) is a common complication in diabetic patients and often leads to the onset of heart failure (HF). Nanoparticle (NP) drug delivery systems are a therapeutic approach that have a potential therapeutic value for improving diabetic HF. This study aims to evaluate the therapeutic effects of NP-loaded sodium-glucose cotransporter-2 (SGLT-2) inhibitors, such as dapagliflozin (DAPA), on HF induced by DCM and elucidate the underlying mechanisms of action. Methods: DAPA nanoscale formulation was prepared. DCM model mice and AC16 cells were treated with different formulations of DAPA (free DAPA, DAPA@poly(lacticco-glycolic acid) (PLGA), DAPA@PLGA-thioketal (TK)-polyethylene glycol (PEG)). The extent of myocardial damage in mice was assessed through staining with hematoxylin-eosin, Masson, and Sirius red. Levels of superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), malondialdehyde (MDA), protein carbonyl (PCO), total iron, and Fe2+ were determined through enzyme-linked immunosorbent assay (ELISA) and UV spectrophotometry. Expression levels of transferrin receptor protein 1 (TfR1) and ferroportin (FPN) proteins in the DCM mouse and high-glucose cell models were measured through Western blot. Reactive oxygen species (ROS) levels in AC16 cells were assessed using fluorescent probes. Changes in mitochondrial damage and membrane potential were evaluated by transmission electron microscopy and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine (JC-1) fluorescent dye. Results: DAPA@NPs have an average diameter of 66.1 nm and a zeta potential of 16.5 mV. At 2.5 mg DAPA, the best encapsulation efficiency (86.11 ± 0.49 %) and load capacity (3.82 ± 2.11 %) were obtained. In the DCM model, DAPA@NPs significantly up-regulated the levels of SOD and CAT in oxidative stress indices, and significantly down-regulated the levels of PCO and MDA (p < 0.001). In addition, DAPA@NPs significantly up-regulate adenosine triphosphate (ATP) levels (p < 0.001) and alleviate mitochondrial damage. Finally, DAPA@NPs inhibit iron death by down-regulating total iron and Fe2+ levels, inhibiting TfR1 expression, up-regulating GSH level, and promoting FPN expression (p < 0.001). Conclusions: DAPA@NPs may treat diabetic HF by inhibiting myocardial fibrosis, reducing oxidative stress, preserving mitochondrial function, and regulating cardiac iron metabolism. |
Keywords: Dapagliflozin, nanocarrier, diabetic cardiomyopathy, myocardial fibrosis, oxidative stress. |
Publication date: 8th April 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 20-32 (PDF file) |